SAT化学练习:Energy Diagrams
来源:未知 发布时间:2014-08-13 09:31 点击:
次
We know that in order for a reaction to occur, reactant molecules must collide and that both an increase in the concentration of reactant molecules and an increase in the temperature of the system can cause an increase in reaction rate. But
We know that in order for a reaction to occur, reactant molecules must collide and that both an increase in the concentration of reactant molecules and an increase in the temperature of the system can cause an increase in reaction rate. But it takes more than just a regular collision to cause a chemical reaction to occur—in fact, only a very small fraction of collisions that occur in the solution lead to a reaction. This is true for two reasons. First of all, for a reaction to occur, the colliding molecules must be oriented in exactly the correct way: they must be oriented in suitable way for the product molecule bonds to be formed. Second, the two molecules must collide with sufficient energy to overcome the activation energy of the reaction. The activation energy is defined as the minimum energy needed to initiate a chemical reaction, and it is symbolized by Ea.Now let’s talk about the energy diagram below.
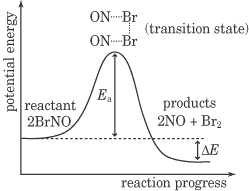
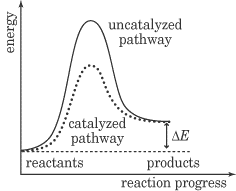
This energy diagram is a graph of the progress of a chemical reaction, versus the total energy of the system. The reactant in this case is BrNO, and the products are NO and Br2. As you can see, after the reaction occurs, the energy of the system is lower than it was before the reaction. This energy diagram shows an exothermic reaction, one in which energy is given off. In the energy diagram for an endothermic reaction, the energy of the products would be higher than that of the reactants. In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products. This state is also known as an activated complex. The figure below shows the energy diagram for a reaction in the presence of a catalyst and in the absence of a catalyst. As you can see, the catalyst has decreased the activation energy of the reaction, which means that more molecules are able to surmount it and react.













 (孙老师)
(孙老师)



